Ensure Compliance.
Optomize Performance.
Make smarter asset decisions.
Download your free guide to selecting the right EAM solution for your organization.

Why Download This Guide?
In highly regulated life sciences environments, the right Enterprise Asset Management (EAM) system isn’t just a nice-to-have—it’s essential. This guide walks you through everything you need to know to evaluate, select, and implement a compliant, scalable, and efficient EAM solution that supports GMP, GLP, and FDA 21 CFR Part 11 requirements.
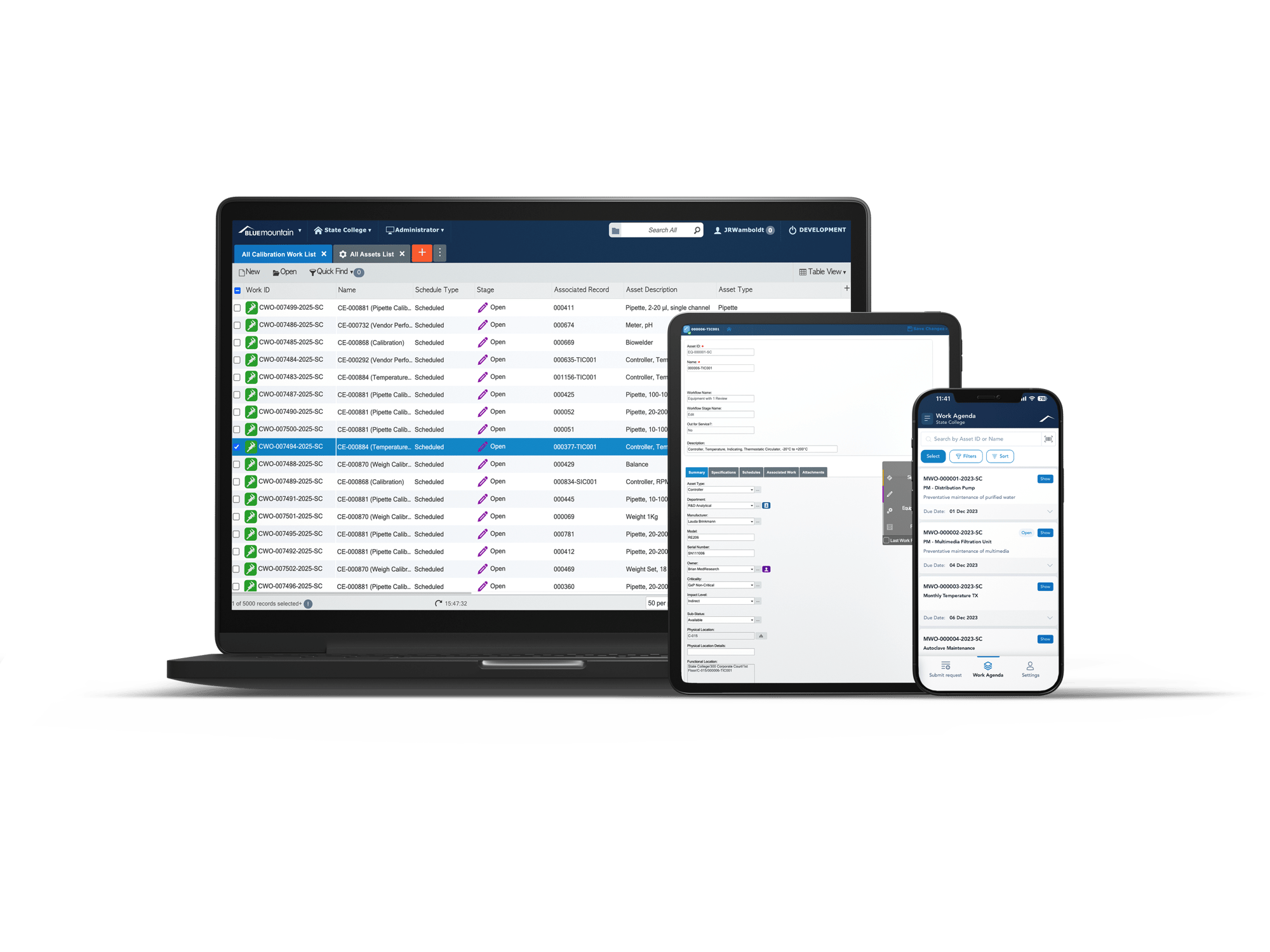
What You'll Learn:
- How EAM drives compliance, data integrity, and operational uptime.
- What makes EAM needs in life sciences unique from other industries.
- Key features to look for in a life sciences-specific EAM platform.
- Best practices for vendor evaluation and implementation planning.
- A practical checklist to prepare your organization for a new EAM system.
Who It's For:
- Facilities and maintenance managers
- Quality and compliance professionals
- IT leaders and system administrators
- Operations and manufacturing teams in pharma, biotech, and med device companies
